Supply high-quality Coenzyme q10
- Categories:News
- Time of issue:2022-11-02 10:30
Supply high-quality Coenzyme q10
- Categories:News
- Time of issue:2022-11-02 10:30

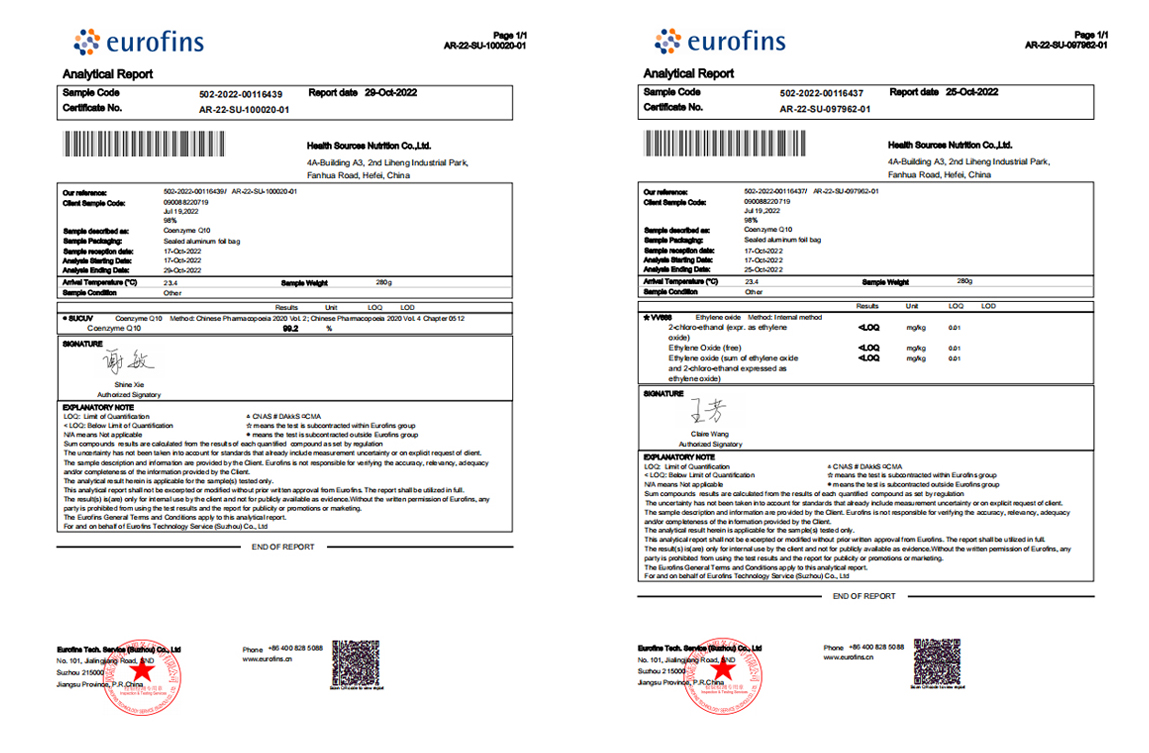
Our coenzyme Q10 passed the quality test of Eurofins, we provide full specification of Co Q10.
| Product and Specification | Characteristics and Applications |
| Coenzyme Q10 98.0~101.0% |
Yellow to orange crystalline powder. Soluble in ether such as trichloromethane and acetone; very slightly soluble in dehydrated alcohol; practically insoluble in water. Suitable for softgels. |
| Coenzyme Q10 10.0% CWD |
Yellow to orange yellow free flowing powder; Easily dispersed in cold water 15°C. Suitable for capsules, tablets and beverages |
| Coenzyme Q10 20.0% CWD | |
| Coenzyme Q10 40.0% CWD |
Yellow to orange yellow free flowing powder; Mostly dispersed in cold water 15°C. Suitable for capsules, tablets. |
We are striving to be a sustainable supplier for you.


NEWS

CONTACT US
Add:4A-Buliding A3, 2nd Liheng Industrial Park,Fanhuan Road,Hefei 230092,China
TEL: (+86) 551 62828690
Fax: (+86) 551 62828697
Email: sales@health-sources.com
Copyright © HEALTH SOURCES NUTRITION CO., LTD.
Our website contains material and information intended for B2B customers, suppliers and distributors, and is not intended as information to the final consumers. The statements on this website have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. In developing claims for a food, beverage or supplement product label, manufactures should seek guidance to assure compliance with the appropriate regulatory authority.




