PROPORTIONAL EXTRACT VS. STANDARD EXTRACT
In the plant extract industry, proportional extracts (Ratio Extract) and standardized extracts (Standardized Extract) are two common types of products, with the core difference lying in the dimension of quality control. Regarding the question "How to understand the extraction ratio in standardized extracts," it is necessary to analyze this in conjunction with the definitions, technical logic, and industry practices of both types.
PROPORTIONAL EXTRACT (RATIO EXTRACT)
Definition: The core indicator is the mass ratio of raw material to extract, reflecting the concentration degree of the extraction process without involving the quantification of active ingredients.
For example, a 10:1 proportional extract means "10 kilograms of raw material (such as turmeric rhizome) yields 1 kilogram of extract after extraction," which only indicates the concentration multiple of the raw material without specifying the content of curcumin and other components.
Characteristics:
- Simple process, lower cost, suitable for scenarios where high precision of ingredients is not required (such as traditional herbal medicines, food flavoring).
- The content of active ingredients may fluctuate due to factors like the origin of the raw material, harvest season, extraction process, etc., resulting in significant batch-to-batch variations.
STANDARDIZED EXTRACT
Definition: A standardized extract is defined by the content of its active ingredient (or characteristic component) as the core indicator. The target component content is controlled through processing to meet specific standards, without mandating a specific ratio of raw material to extract.
For example: A standardized turmeric extract with 30% curcumin content meets the standard as long as the curcumin proportion in the final extract is 30%, regardless of the amount of raw material used.
Characteristics:
- Precise control of components through chromatographic analysis (such as HPLC), resulting in high batch-to-batch consistency. It is suitable for fields like dietary supplements and pharmaceuticals where efficacy is strictly required.
- May imply a certain extraction ratio (for example, high-purity extracts typically require more raw material to concentrate), but the ratio is not a mandatory labeling item.
HOW TO UNDERSTAND THE "EXTRACTION RATIO" IN THE STANDARD REFERENCE SUBSTANCE?
The Essence of Extraction Ratio: The Concentration Relationship between Raw Material and Extract
In standard extracts, the "extraction ratio" (if labeled) typically serves as an auxiliary indicator that reflects the relationship between the amount of raw material input and the output of extract in the production process.
For example:
- Labeled as "5:1 standard extract (curcumin 30%)": This indicates that 5 kilograms of raw material are used to produce 1 kilogram of extract, and the curcumin content in this extract is 30%.
- Logical Relationship: Extraction ratio=mass of raw material÷mass of extract. The larger the numerical value, the higher the degree of concentration (e.g., 10:1 is more concentrated than 5:1).
Relationship between Extraction Ratio and Active Ingredient Content
-Non-determinative factor: The extraction ratio only reflects the concentration factor and does not directly determine the active ingredient content.
For example:
-Raw material A (with a curcumin content of 5%) is extracted using a 5:1 ratio, and the curcumin content in the extract is approximately 5%×5=25% (theoretical value, without considering process losses).
-Raw material B (with a curcumin content of 8%) is extracted using a 5:1 ratio, and the curcumin content in the extract is approximately 8%×5=40%.
Therefore, under the same extraction ratio, the ingredient content of the raw material itself directly affects the active ingredient content of the final extract.
The core of a standardized extract is the standardization of active ingredients: by adjusting the extraction process (such as solvent usage, concentration level, purification steps), regardless of the raw material ratio, the target component is ultimately adjusted to a specified content (for example, forcibly adjusting curcumin to 30%).
Annotation Logic in Industry Practice
Most standard extracts do not mandate the annotation of extraction ratios:
Companies are more focused on the content of active ingredients (such as "Curcumin 30%"), while the extraction ratio may vary due to differences in raw material batches, process optimization, etc., and is only used as a reference.
Annotation Scenarios:
Some companies, to reflect the input amount of raw materials, will annotate both the ratio and the ingredient content (such as "10:1, Curcumin 5%"), implying "high ratio = high raw material input=potentially higher active ingredients," but this needs to be judged in conjunction with actual test data.
Traditional herbal extracts (such as traditional Chinese medicine) may retain the habit of annotating "ratios," but this needs to be accompanied by the detection of active ingredients.
DIFFERENCES AND APPLICATION BETWEEN PROPORTIONAL EXTRACTS AND STANDARD EXTRACTS
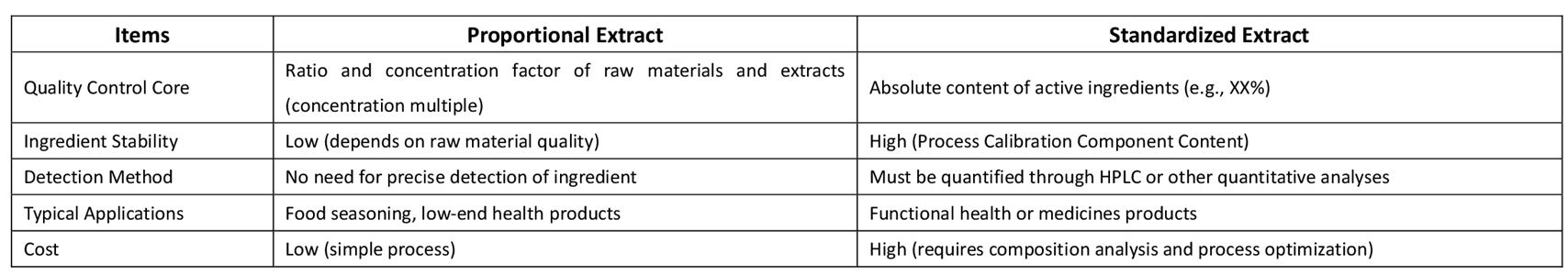
Example:
If stable curcumin coloring or functional effects are required for the production of solid beverages, a standard extract (such as curcumin 5%-10%) should be selected to ensure consistent composition across each batch.
If only the "addition of turmeric raw material" needs to be reflected, with no strict requirements on efficacy, a ratio extract (such as 5:1) can be chosen, but the risk of composition fluctuation must be accepted.
COMMON MISCONCEPTIONS AND PRECAUTIONS
Misconception 1:
The higher the extraction ratio, the higher the content of active ingredients: Incorrect.
The ratio only reflects the concentration factor. If the content of active ingredients in the raw material is low, the content of active ingredients in a high-ratio extract may still be lower than that in a low-ratio product (for example, if the curcumin content in the raw material is 1%, the content in a 10:1 extract is approximately 10%; if the curcumin content in the raw material is 5%, the content in a 5:1 extract is approximately 25%).
Misconception 2:
Standard extracts must be labeled with the extraction ratio: Incorrect.
International mainstream standards (such as the US FDA and the European EFSA) focus on the content of active ingredients as the core, and the extraction ratio is not a mandatory requirement. It is only voluntarily labeled by some companies.
Procurement Recommendations:
Clarify Requirements: For functional products, prioritize standard extracts and focus on the Certificate of Analysis (COA) for active ingredient testing; for cost-sensitive products, consider ratio extracts, but request raw material composition data.
Beware of "Pseudo-Standards": Some companies may misrepresent "high ratio" as "high content," so it is essential to verify third-party test results.
SUMMARY
The core of standard extracts is the standardization of active ingredients. The extraction ratio (if labeled) is merely a reference for the input amount of raw materials and does not directly determine product quality.
Proportional extract focus on the concentration factor and are suitable for scenarios where less precision of ingredients is required, but they carry the risk of batch differences.
When making a choice, it is necessary to consider the quality requirements of the application scenario, cost budget, and regulatory requirements, prioritizing the detection data of active ingredients as the basis for judgment rather than the single extraction ratio.
MAIN LAWS AND REGULATIONS THAT P.E SHOULD COMPLY WITH IN THE EUROPEAN MARKET
[§] New Food Regulation (EU 2015/2283): If a plant extract is identified as a novel food, meaning it was not widely consumed in the European market before May 15, 1997, it must comply with the new food regulations. [§] Companies need to conduct a safety assessment and submit an application to the relevant EU authorities, demonstrating that the plant extract is safe under the intended conditions of use. Additionally, aspects such as its composition, production process, scope of use, and labeling must all comply with regulatory requirements.
[§] Directive 2001/83/EC on Traditional Herbal Medicinal Products: Traditional herbal medicines need to be registered according to this directive, and a history of safe use for more than 30 years is required to be exempted from some clinical trials. [§] For example, chamomile can be sold directly as a herbal medicine as long as it can be proven to have a history of use in Europe for more than 30 years.
[§] Regulation (EC) No 1333/2008 on food additives: If plant extracts are used as food additives, they must undergo a safety assessment by the European Food Safety Authority (EFSA) and obtain approval before use. They must also comply with regulations regarding maximum usage levels, areas of use, etc.
[§] EU Register of Nutrition and Health Claims (EC 1924/2006): For plant extracts used as dietary supplements, health claims must undergo EFSA scientific assessment and be listed in this register before they can be used. The label must accurately state the ingredients, usage instructions, and information about unsuitable populations, and no disease treatment claims can be made.
[§] Regulation (EU) No 1925/2006 of the European Parliament and of the Council on the addition of vitamins and minerals and of certain other substances to foods: When plant extracts are added as certain substances in food supplements, they need to comply with the requirements of this regulation, such as the relevant provisions for catechins in green tea extract, etc.
QUALITY AND SAFETY STANDARDS
1.Active Ingredient Content:
Different plant extracts have specific active ingredients. For example, Ginkgo biloba extract has a specified content range for ginkgo flavone glycosides and terpene lactones, generally requiring that ginkgo flavone glycosides be ≥24% and terpene lactones be ≥6%, to ensure the efficacy of the product.
2. Contaminants Limits:
2.1 Heavy Metals: The limits for heavy metals such as lead, mercury, cadmium, and arsenic are strictly regulated, typically with Lead (Pb)≤3.0mg/kg; Arsenic (As)≤2.0mg/kg; Cadmium (Cd)≤1.0mg/kg and Mercury (Hg)≤0.1mg/kg
2.2 Pesticide Residues: Compliance with the European Union pesticide residue standards (EC) No 396/2005 is required. Different plant extracts have specific limits based on their intended use and source materials. For example, the total amount of pyrethroid pesticides in certain plant extracts must not exceed 0.05mg/kg.
2.3 Microbial Limits: Must meet relevant microbial standards, such as total bacterial count≤1,000CFU/g (for solid extracts) or ≤100CFU/mL (for liquid extracts), total mold and yeast count ≤100CFU/g or mL, and the absence of coliform bacteria, Salmonella, and other pathogenic bacteria.
3. Production Process Standards
3.1 Raw Material Selection: Adhere to Good Agricultural and Collection Practices (GACP). Plant materials should be sourced from suitable cultivation areas with uncontaminated soil, water sources, and air. Organic cultivation is preferable to avoid excessive use of pesticides and fertilizers, ensuring stable quality of raw materials and absence of harmful substance contamination.
3.2 Extraction Methods: Select appropriate extraction methods based on plant materials and target components, such as solvent extraction, steam distillation, supercritical fluid extraction, etc. The extraction process must strictly control parameters such as temperature, pressure, and time to ensure the consistency and stability of the extract quality.
3.3 Production Environment: Production facilities must comply with Good Manufacturing Practice (GMP) requirements. Production workshops should be clean and hygienic, equipped with dust-proof, insect-proof, and rodent-proof facilities. Equipment should be regularly cleaned and maintained to ensure no foreign matter contamination or cross-contamination during the production process.
4. Safety Assessment Criteria
4.1 Toxicological Assessment: New plant extracts or plant extracts with new uses require toxicological studies, including acute toxicity, subchronic toxicity, chronic toxicity, genotoxicity, reproductive toxicity, and other tests to evaluate potential health risks.
4.2 Quality Control: The European Food Safety Authority (EFSA) and the European Medicines Agency (EMA) are responsible for the safety assessment and regulation of plant extracts in the food and pharmaceutical sectors, respectively. Companies must submit detailed information to the relevant authorities, including plant source, extraction method, compositional analysis, toxicological data, etc., and can only be marketed after passing the assessment.
ETO, SUM PAHS 4 AND PYRROLIZIDINE ALKALOIDS
1. ETO (Ethylene Oxide)
Prohibition: The European Union has banned the use of ethylene oxide in food since 1991. Since July 2021, all products in the EU containing ethylene oxide above the limit of quantification of 0.1mg/kg must be withdrawn from the market.
Limit: The EU has set the limit of detection for ethylene oxide at 0.05mg/kg, which includes ethylene oxide and its metabolite 2-chloroethanol (expressed as ethylene oxide).
ETO≤0.05 mg/kg
Detection method: GC-MS/MS
2.Sum PAHs 4
Definition: Refers to the sum of benzo(a)pyrene, benzo(a)anthracene, benzo(b)fluoranthene, and chrysene.
Relevant Regulations: The European Commission Regulation (EU) 2023/915 sets limits for Sum PAHs 4 in food.
Limits: Different foods have different limit standards, for example, the Sum PAHs 4 limit for cocoa beans and derived products is 30 micrograms/kilogram; for coconut oil, it is 20 micrograms/kilogram; for other oils, it is 10 micrograms/kilogram; for smoked meat and meat products, it is 12 micrograms/kilogram; for smoked fish and fishery products, it is 12 micrograms/kilogram; for smoked mollusks, it is 35 micrograms/kilogram; for infant foods and infant formula, it is 1 microgram/kilogram; for traditionally smoked meat and fishery products (produced and sold in specific countries such as Ireland and Croatia), it is 30 micrograms/kilogram; for dried herbs and spices, and plant powders used for preparing beverages, it is 50 micrograms/kilogram; for banana chips, it is 20 micrograms/kilogram.
*Sum PAHs 4≤50ppb (Benzo(a)pyrene≤10ppb)
Detection Method: GC-MS/MS
* Sum PAHs 4 (Benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene)
3. Pyrrolizidine Alkaloids
Toxicity: Widely distributed in the plant kingdom, especially in plants of the Asteraceae, Fabaceae, and Boraginaceae families, possessing genotoxicity.
Related Incidents: In 2017, Belgium detected pyrrolizidine alkaloids in food supplements imported from the UK and France and reported it through the EU's Rapid Alert System for Food and Feed. The German Federal Institute for Risk Assessment found that some tea and herbal tea products contained relatively high levels of pyrrolizidine alkaloids.
Limits: Currently, there are no unified and clear limit standards for pyrrolizidine alkaloids in food and health product ingredients in Europe. However, due to its toxicity, monitoring and control are carried out on potentially contaminated raw materials to minimize their presence in the ingredients.
Hot Tags:
PREVIOUS:








